Sheila Madan
What's the difference between Biobrume's product and the HOCl we can make at home from portable HOCl kettle generators?
Until recently it has not been possible to create hypochlorous acid in a stable solution with a usable shelf life. Hypochlorous acid generated from dissolving dry calcium hypochlorite has a shelf life of approximately four hours, therefore has not been suitable for use in pharmaceutical, healthcare or any other commercial environment unless it's generated on site.
Advances in chlorine chemistry have allowed Biobrume to bring to market a stabilised HOCl solution, at a pH of 4–6 so all available chlorine is in the form of hypochlorous acid. This product has a half-life of 12 months, or an effective shelf life of 18 months, and is referred to as "stabilised" hypochlorous acid.
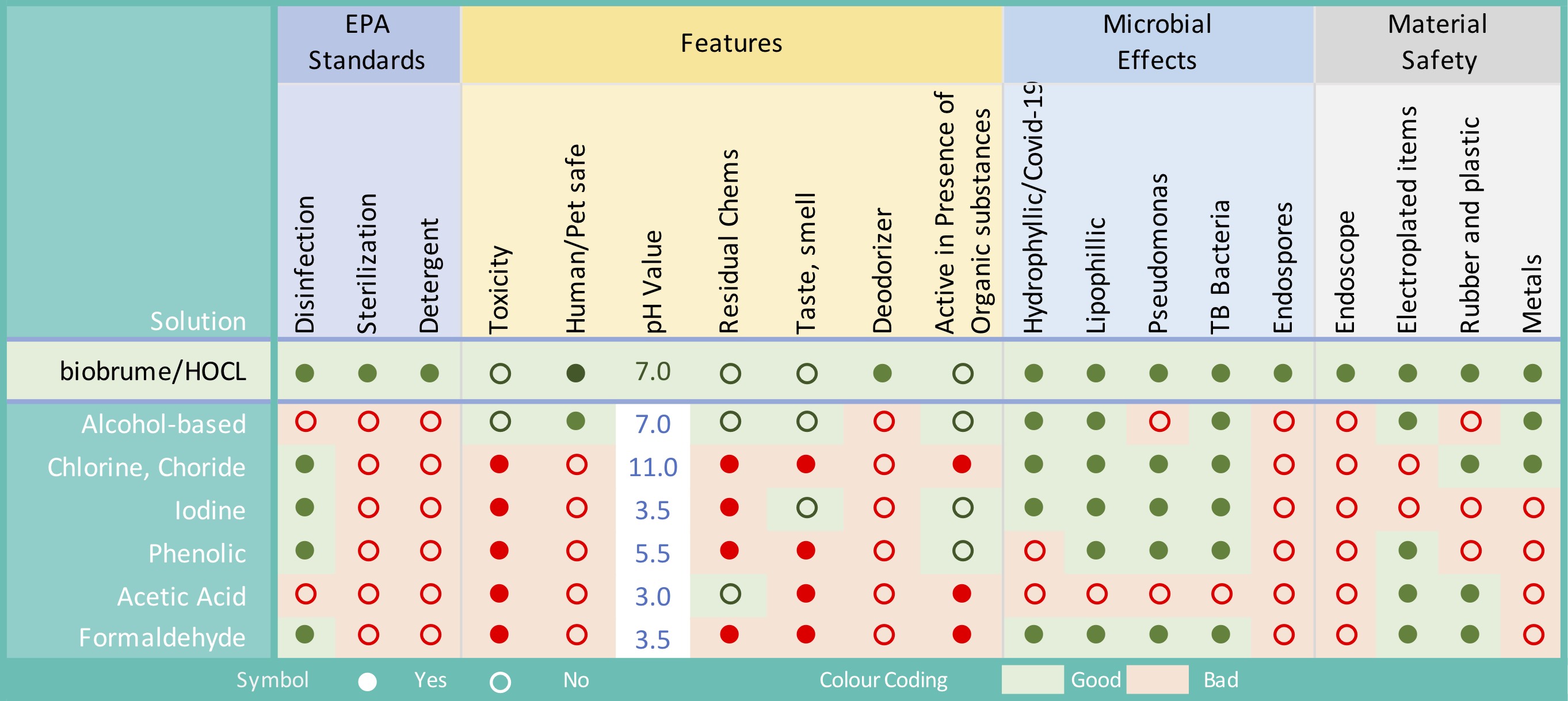
Stablized HOCl is more efficacious and faster acting than the equivalent sodium hypochlorite (bleach) product while also demonstrating high material compatibility. HOCl displays complete kill against spores, bacteria and viruses in both clean and dirty conditions against a modified EN 13697 surface tests in one minute. This is a significant increase when compared with sodium hypochlorite in both the log reduction achieved and the kill rate.
Various micro-organisms have been tested including a particular resistant house isolate which was “typed” as P. glycolyticus. Test work was also repeated against the newest version of EN 16506 for fungi and moulds, which has a requirement for >75% of the A. niger (now A. brasiliensis) spores to be in the form of “spiny spores” which are more resistant.
HOCl is the perfect weapon to fight germs. It hits hard against pathogens like Methicillin-Resistant Staphylococcus Aureus and Pseudomonas Aeroginosa. Yet this powerful weapon is 100 percent safe for humans, chemical free, non-toxic and all-natural. That’s an impressive combination. It has been used in the medical field for over a century. Before antibiotics were available, HOCl was used to irrigate and disinfect wounds in World War I. It is now used in everyday settings including daycare centers, hospitals, and even produce sections in grocery stores.
Due to its low pH and available chlorine concentration, the product also carries no hazard and thus requires no specialised PPE or disposal consideration. It is non-corrosive and current testing shows good compatibility with cleanroom materials.
Efficacy of Biobrume HOCl
Stability of Hypochlorous Acid
Source
Despite compelling evidence for HOCl’s use as a sanitation resource, commercialization of environmental applications has been slow to materialize. Carefully controlled pH adjustment of hypochlorite solutions results in an equilibrium shift towards a predominance of HOCl. However, this has proven difficult to do at scale for generating a consistent product without contamination by molecular chlorine (Cl2), trichloride, hypochlorite or chlorate/chlorite ions. Additionally, solutions prepared in this way without careful consideration of materials and process controls commonly show an instability that undermines the advantages of HOCl.
Commercial manufacture of HOCl has become possible through electrolysis of sodium chloride brine (Agriculture Marketing Service, 2015). Electrolysis isolates HOCl and NaOH at the anode and cathode respectively. Harvesting the anode region results in solutions enriched for HOCl but prone to rapid decay into chlorate/chlorite and hypochlorite mixtures. Claims for the useful life of such products vary from hours to weeks, imposing practical constraints on its use. Widespread applications in institutional settings have therefore been limited by the need for on-site production equipment and the associated electrical power, personnel, and process needs.
Stabilization of electrolytic preparations of HOCl can be encouraged by judicious use of additives including buffers, metal cations, and periodate (for further discussion see US patent 7393522 2B), and stable pharmaceutical formulations have been successfully created by controlled pH manipulation with the addition of HCl. Products made by both methods have met with FDA/EPA regulatory approvals permitting shelf life claims of up to two years (for FDA 510(k) device clearances see: K113820 (Novabay); K042729 (Oculus Innovation Sciences); K123071, K093697 (Puricore).
Conclusions and Significance
Innate resistance to infection in humans depends on the production of HOCl at the front line of defense against microbial invasion. Nature has utilized this compound in immune defense systems across the vertebrate sub-phylum and speaks well to its sustained power, speed, spectrum of action and reliability. It also accounts for the biocompatibility of HOCl, which must be swiftly neutralized in vivo to avoid adverse physiological effects.
Being able now to apply HOCl in the service of infection control practices is a major advance of the 21st century. Adoption of HOCl as a stable disinfectant may also solve one of the most troubling and persistent gaps of traditional sterilization procedures – prions. Reports have documented the increasing evidence that subtypes of dementia, including Alzheimer’s, may have an infectious component that currently looks like a prion-related protein. Having a disinfectant that can eradicate that risk from surgical instruments and medical facilities is a timely finding, and a welcome advance in infection control technology.

